INTRODUCTION
The benefits of regional anesthesia (RA) for acute pain control have been well-documented to include decreased opioid consumption, increased patient satisfaction, and decreased cost for both hospital and patient.1,2,3,4,5 The benefits of using United States (US) in regional anesthesia include faster analgesic onset, shorter procedure duration, lower dose of local anesthetic (LA) used, decreased incidence of local anesthetic systemic toxicity (LAST), and lower incidence of direct needle trauma.5,6,7,8,9,10
When nerve blocks fail or are ineffective, there is usually an increased dependence on opioids which is associated with increased 30-day readmittance rate, opioid use dependance, and increased length of hospital stay, as well as other complications.5,11 Whether the nerve or nerve plexus is partially or fully anesthetized, concern historically exists about the risk of direct needle trauma with a rescue block. Nerve stimulation is ineffective in this setting, but it is unclear if this concern is well-founded in this setting of US-guidance.12 In many ways, performing a ‘rescue’ nerve block after previously failed or partially-functioning nerve block can be compared with the risks of performing RA while a patient is heavily sedated or under general anesthesia. While US has been shown as an effective tool for performing nerve blocks for patients under general anesthesia and in heavily sedated patients, one cannot rely on patient feedback to recognize close needle approximation to the nerve in this situation.13,14
In the same way, US can be used to perform a rescue nerve block at a site where LA has been previously administered. US-guidance allows for real-time needle visualization as well as opportunity to perform techniques that do not require close needle approximation. These differences, along with a new appreciation for the negative consequences of opioids, may represent a much different risk benefit ratio to perform rescue blocks than in previous eras. This retrospective study examines the efficacy and safety of US-guidance in performing rescue nerve blocks in an acute trauma patient population.
MATERIALS AND METHODS
Methods
Institutional review board (IRB) approval was obtained through University of Tennessee Health Science Center before the study began (IRB acceptance 21-08120-XP). Patients were selected from Regional One Health, a 337 bed level 1 trauma center. Inclusion criteria consisted of being over the age of 18 at the time of admission and having at least one failed nerve block that was replaced during a single in-patient visit. This retrospective study examines the efficacy and safety of US-guidance in performing rescue nerve blocks in an acute trauma patient population. Once selected, patient data was de-identified and collected into a password protected Excel sheet for analysis.
All continuous peripheral nerve blocks (CPNB’s) utilized were grouped into high-risk (brachial plexus, saphenous, lateral femoral cutaneous, popliteal sciatic) and low-risk (erector spinae plane (ESP), subpectoral, serratus, rectus sheath, subgluteal sciatic, femoral and fascia iliaca) categories based on the need for close needle to nerve proximity required to perform the CPNB. To determine if a CPNB was functioning to some degree, and therefore represented a potentially increased risk of direct needle trauma, daily acute pain service (APS) progress note assessments were reviewed. Degree of objective evidence of appropriate loss of cold sensation or numbness (and motor weakness for extremities) was considered a partially or fully functioning CPNB, while denial of loss of cold sensation (and fully intact motor function for extremities) was considered a non-functioning CPNB. In situations where a physical exam was not possible such as with amputations, increased opioid consumption and increased patient-reported pain were utilized to identify functioning and non-functioning CPNB’s. All opioids consumed 24-hours prior to and after initial and rescue nerve blocks was collected and converted to morphine milli-equivalents (MMEs) based on standard conversion ratios.
Statistical Analysis
A sample size of 55 patients was identified. Data was imported to Tableau for grouping, Excel was used for statistical analysis, and GraphPad was used to generate figures.
RESULTS
Patient’s demographics and risk factors associated with an increased risk of nerve injury are shown in Table 1. Of the 55 patients examined, 5 had two CPNB rescue block sites, so these were included to bring the total rescue blocks sites performed up to 60. While 10 patients had their rescue site re-blocked multiple times bringing the total number of rescue blocks performed to 74, conditions were similar to the initial rescue block in each case, therefore, only data on the initial rescue nerve block data was collected.
| Table 1. Patient Characteristics (n=55) |
|
n
|
|
| Mean age in years (range) |
45.7 (18-78)
|
| Mean weight in kg (sd) |
93.8 (30.4)
|
| Male/female |
36/19
|
| Morbid obesity, n (%) |
13 (24%)
|
| Diabetes mellitus, n(%) |
9 (16%)
|
| Pre-existing neurological disorder, n (%) |
5 (9%)
|
There was no statistically significant difference in the time required to perform initial nerve blocks compared to rescue nerve blocks overall (p=0.633), and there was no significant difference in time to perform initial and rescue blocks for high nerve blocks (p=0.65). There was, however, a statistically significantly reduction in the time required to perform rescue nerve blocks compared to initial nerve blocks in the low-risk group (p=0.019). Initial and nerve blocks remained in place for an average of 5.2-days before replacement. Rescue nerve blocks remained in place for an average of 6.5-days, thus the average combined number of catheter days was 11.52-days. The average and maximum number of combined catheter days for each nerve block type is listed in Table 2.
| Table 2. Time to Perform Initial and Rescue Nerve Blocks with Average and Maximum Number of Catheter Days |
|
# of Initial Nerve Blocks Performed
|
Time to Perform Initial Block (min) |
Time to
Perform Rescue Block (min) |
Average Initial Block Catheter Days |
Average Rescue Block Catheter Days |
Average
Combined Catheter Days |
Maximum
Combined
Catheter Days
|
| High-Risk |
31
|
9.7 |
11.1 |
3.9 |
5.6 |
9.5 |
24
|
| Brachial plexus |
10
|
11.2 |
9.8 |
5.3 |
4.9 |
10.2 |
25
|
| Lat Fem Cutaneous |
4
|
11.5 |
9.3 |
5.7 |
4.7 |
10.4 |
13
|
| Popliteal Sciatic |
16 |
11.6 |
12.9 |
4.7 |
5.6 |
10.3 |
24
|
| Saphenous |
1
|
7.0 |
13.0 |
0 |
13.0 |
13.0 |
13
|
| Low-Risk |
29
|
14.5 |
7.9 |
4.9 |
7.3 |
12.2 |
27
|
| ESP |
13
|
13.3 |
12.6 |
4.4 |
6.5 |
10.9 |
21
|
| Femoral |
1
|
12.0 |
9.0 |
2 |
10.5 |
12.5 |
12.5
|
| Fascia Iliaca |
5
|
16.4 |
9.6 |
4.4 |
7.2 |
11.6 |
23
|
| Serratus |
5
|
13.0
|
8.6 |
8.6 |
4.2 |
12.8 |
28
|
| Subgluteal Sciatic |
3
|
18
|
9.7 |
6 |
10.7 |
16.7 |
27
|
| Subpectoral |
2
|
23.5 |
10.5 |
4.0 |
3.0 |
7.0 |
7
|
Both the initial nerve block and rescue block procedure notes were checked for noted difficulty during the procedure. There were only 3 (5%) patients that had both a complex original block and rescue block, while there were 12 and 13 patients that had noted complexity in the original and rescue blocks, respectively. The majority of the listed reasons for being a difficult procedure in both the initial and rescue blocks was either due to the presence of subcutaneous air or difficult patient positioning due to pain from the existing injury. Reasons for replacement of functioning CPNB’s were due to either accidental catheter removal because catheters would be in the surgical field or as a preventive measure against infection risk. Failed or inadequate CPNB’s occurred either immediately or over time in 21 of 74 occurrences. In 2 high-risk and 2 low-risk CPNB’s, the reason for replacement was not clearly described in the APS notes. There was no significant correlation between the type of nerve block performed and the reason a rescue was needed (r=0.48, p=0.27). Table 3 shows the reasons for nerve block replacement for high- and low-risk sites.
Sensory and motor examinations immediately prior to rescue nerve blocks were evaluated and are summarized in Figure 1. When bandages or dressings interfered with a proper physical exam or there was a lack of an extremity to evaluate, this is listed as ‘unable to assess’. A partially or fully functioning CPNB was confirmed 98% of the time that rescue blocks were performed. Only 3 patients denied a decrease in cold sensation prior to rescue blocks. Of the 23 patients where the initial nerve block was unable to be assessed by physical exam, 6 reported an increase in pain between the initial block and pre-rescue physical exam which prompted their rescue block. Pain resolved after their rescue nerve block. The remaining 17 patients had nerve blocks rescued without evidence of failed CPNB or inadequate analgesia for previously described reasons in Table 3.
| Table 3. Reason for Rescue Nerve Block |
|
Reason for Rescue Block
|
High-Risk |
Low-Risk
|
| Extended utilization |
22
|
13
|
| Catheter dislodged or accidently removed |
6
|
6
|
| Infection prevention measure |
6
|
1
|
| Surgery (catheter in surgical field) |
10
|
6
|
| Failed or inadequate analgesia |
7
|
14
|
| Incomplete or unsuccessful block |
6
|
13
|
| Leaking catheter |
1
|
1
|
| Other |
2
|
2
|
Figure 1. Functional Evaluation of CPNB Just Prior to Rescue Block

Opioid consumption was examined daily, 24-hours before the initial nerve block was performed, 24-hours after the initial nerve block, before the rescue nerve block, and after the rescue nerve block. Figure 2 shows opioid consumption by time relative to the initial and the rescue blocks. There was a significant decrease in average opioid consumption immediately after the initial block (p=0.033), as well as a significant decrease between the pre-initial block average and the post-rescue average (p=0.027). There was no significant difference between average opioid consumption immediately after initial block and post-rescue (p=0.64). When the initial nerve blocks that were replaced due to failure or inadequacy were examined separately, there was a decrease in opioid use following rescue block that did not reach statistical significance (Table 4). As anticipated, a change in opioid consumption did not occur when functioning nerve blocks were rescued in order to extend utilization of the CPNB. Daily evaluation of opioid consumption revealed that 24 (40%) patients reached zero opioid consumption an average of 2.9 (2.2 standard deviation (SD)) days after the initial nerve block was performed as shown in Figure 3.
Figure 2. 24-Hour Opioid Consumption before and after Initial and Rescue Nerve Blocks

Figure 3. Days until Zero Opioid Consumption Following Initial Block

Figure 2 shows the number of blocks grouped into complete failure, partially functioning, or fully functioning based on pre-rescue exam findings. Complete failure was defined as denial of decreased temperature sensation, increase of pain sensation, and increase in opioid consumption. A fully functioning block was defined as confirmed loss of temperature or motor sensation on physical exam, or when unable to assess, both decreased opioid consumption and decrease in pain sensations. The remainder were grouped into partially functioning.
Initial nerve blocks were grouped into complete failure, partially functioning, or fully functioning based on pre-rescue exam findings or new complaints of pain with increased opioid consumption (Figure 4). Fully Functioning nerve blocks were those having a confirmed loss of temperature or motor sensation on physical exam, or when unable to assess, both an ongoing decrease in opioid consumption and a decrease in pain were present. Complete failure of the nerve block was defined when there was no decrease in temperature sensation at all with full motor function on physical exam along with an increase in pain and an increase in opioid consumption. This circumstance was identified for only one patient. The remainder of the nerve blocks were grouped into the partially functioning category which included physical examinations that described some decrease in cold sensation or motor function without an increase in opioid consumption but with some increase in reported pain.
Figure 4. Degree of CPNB Function Prior to Rescue Block

Figure 5. Reduction in Pain Reported After Initial and Rescue Blocks

Subjective data was collected on patient-reported reductions in pain immediately following initial and rescue nerve blocks (Figure 5). There were 39 patients (65%) that reported a reduction in pain immediately after the initial nerve block compared to 34 patients (56%) that reported a reduction in pain immediately after receiving the rescue block.
All APS patients undergo a ‘clamp trial’ prior to discharge to determine if an outpatient CPNB infusion is warranted or when evidence suggests that the CPNB is no longer required. At this time, neurologic injury may be identified once the CPNB has fully worn off. Further, the APS continues to follow outpatients by telephone until their CPNB has been removed at which time, patients can still contact the APS with newly recognized neurologic concerns that arise after the CPNB has resolved fully. Data was collected during the initial hospitalization and in surgical clinic follow-up appointments afterward to determine if there was evidence to suggest lasting injury to nerves that was potentially attributable to the rescue nerve block (Figure 6). There were no surgical or APS notes for any patient where nerve injury was discussed or described during their initial hospitalization. Follow-up clinic visit notes for 36 patients were available for review, and no evidence to suggest lasting damage to nerves that was potentially attributable to the rescue nerve block for any of these patients. For 19 patients, no meaningful information could be found pertaining to their health or wellness after discharge from the initial hospitalization. There was no evidence of minor or major signs or symptoms of LAST in the APS procedure notes or other APS notes in any of the patients in this study.
Figure 6. Follow Up Data Available after Initial Hospitalization

Reviewing the available medical records on 55 patients encompassing 60 rescue nerve block sites and a total of 74 rescue blocks performed, there was no evidence of nerve injury that could be attributed to direct needle trauma or extended exposure of nerves to local anesthetics due to rescue blocks performed on patients with partially or fully anesthetized nerves.
DISCUSSION
The results from this study indicate that by replacing an inadequate CPNB, opioid consumption returns back to the reduced baseline amount immediately present after an initial nerve block. Patient pain ratings are better controlled after both initial and rescue nerve blocks; this is both an indicator of block success and has been shown to correlate with patient satisfaction.5,15
As well, continuing the utilization of a functioning CPNB maintains reduced opioid consumption. This is further supported by a similar number of patients that reported a reduction in pain following initial and rescue nerve blocks. The continued utilization of a CPNB after acute trauma leads to zero opioid consumption in many patients. While not evaluated in this study, replacing a failed nerve block or extending the utilization of a functioning CPNB could lead to additional benefits of lowering opioid consumption such as decreased hospital costs, decreased length of stay and lower 30-day readmission rate.1,16
In this study, most of the rescue nerve blocks were performed after functioning CPNB’s were inadvertently removed, were going to be in the surgical field or to minimize the possibility of infection. Other reasons included poor initial catheter placement, leaking catheter dressings, incomplete analgesia, or other unknown reasons. The benefits related to the therapy provided by the CPNB’s continued safely for two weeks or more in many cases. If a continuous nerve block is functioning well and either needs to be replaced for continuing analgesia, or becomes dislodged due to surgery or accident, this likely represents a higher risk rescue nerve block due to the presence of anesthetized nerves
Alternatively, a block that fails due to incorrect placement, or other situations where there is no local anesthetic is getting to the desired location would represent a complete block failure, and thus a much lower-risk of direct needle trauma with a rescue nerve block. An elevated risk of nerve injury related to performing a rescue nerve block was established in this study by objectively confirming through physical exam and by inference when previously well-controlled pain was reported by patients to have worsened and was accompanied by an increase in opioid consumption. However, out of 55 patients who had 74 rescue nerve blocks performed on 73 partially or fully functioning nerve blocks, 37 were confirmed to have no damage related to the rescue block. The remaining 18 were lost to follow-up, but no evidence of nerve injury was identified in the chart during their hospitalization. This evidence was present for rescue nerve blocks categorized as high and low-risk of direct needle injury in an acute trauma setting in the presence of additional risk factors to nerve injury.
Weaknesses of this study include the retrospective nature of the design, the relatively few cases presented and the loss to follow-up of several patients. Further, the clamp trial process may have be able to identify some patients with evidence of neurologic injury, however, full resolution of the CPNB does not always occur prior to restarting the CPNB when pain returns, and the APS may not continue following some patients long enough after discontinuing a CPNB to identify evidence of subtle injury. Patients lost to follow-up may believe that ongoing neurologic symptoms are expected and defer reporting these symptoms. These issues may be a reason for the lack of recognized neurologic injury.
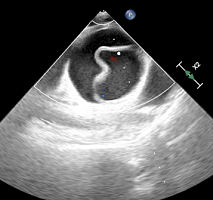
While the risks of direct needle trauma when performing a rescue nerve block are not to be understated, US-guidance provides ways to minimize this through direct needle visualization and by performing nerve blocks distant to nerves that were unavailable with previous nerve-localization techniques. For example, when performing femoral or fascia iliaca nerve blocks, a ‘plane approach’ can be utilized similar to an EPS block. In this scenario, the needle is aimed a distance away from the femoral nerve under the fascia iliaca, therefore not requiring the needle to come in close proximity with the nerve.12 Additionally, in many cases, the presence of previously administered LA can actually provide a hypoechoic ‘cushion’ surrounding the nerve, making visualization of the target nerve easier and providing a safe place to deposit local anesthetic that is not immediately adjacent to the nerve. In the case of a popliteal sciatic nerve block, without touching the common peroneal and tibial nerves as shown in the image below (Figure 7). This ‘hypoechoic cushion’ and the presence of previously administered LA
Figure 7. Hypoechoic Cushion before and After Local Anesthetic Injection

LA may be one reason that low-risk rescue nerve blocks were performed more quickly than the initial nerve blocks.
CONCLUSION
US-guided rescue nerve blocks have been shown to be safe and effective in reducing and keeping opioid consumption low, as well as in improving pain. While there are risks of performing rescue nerve blocks, 74 rescue nerve blocks were performed in 55 trauma patients without evidence of adverse outcomes related to direct nerve injury in the presence of partially or fully anesthetized sensory nerves. Larger prospective studies are needed to confirm the initial findings of this study.
FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.